Solving the Puzzle of Unusual Excited-State Proton Transfer in 2,5-Bis(6-methyl-2-benzoxazolyl)phenol
Jacek Dobkowski, Michał Kijak, Sylwester Gawinkowski, Elena Karpiuk, Mariusz Pietrzak, Igor V. Sazanovich, and Jacek Waluk
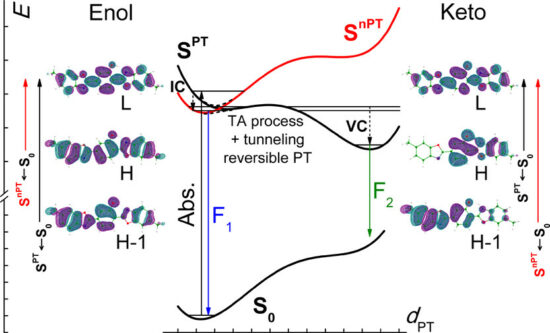
2,5-Bis(6-methyl-2-benzoxazolyl)phenol (BMP) exhibits an ultrafast excited-state intramolecular proton transfer (ESIPT) when isolated in supersonic jets, whereas in condensed phases the phototautomerization is orders of magnitude slower. This unusual situation leads to nontypical photophysical characteristics: dual fluorescence is observed for BMP in solution, whereas only a single emission, originating from the phototautomer, is detected for the ultracold isolated molecules. In order to understand the completely different behavior in the two regimes, detailed photophysical studies have been carried out. Kinetic and thermodynamic parameters of ESIPT were determined from stationary and transient picosecond absorption and emission for BMP in different solvents in a broad temperature range. These studies were combined with time-dependent- density functional theory quantum-chemical modeling. The excited-state double-well potential for BMP and its methyl-free analogue were calculated by applying different hybrid functionals and compared with the results obtained for another proton-transferring molecule, 2,5-bis(5-ethyl-2-benzoxazolyl)hydroquinone (DE-BBHQ). The results lead to the model that explains the difference in proton-transfer properties of BMP in vacuum and in the condensed phase by inversion of the two lowest singlet states occurring along the PT coordinate.